R&D
For a better future SCM Lifescience will do its best
Core Technology
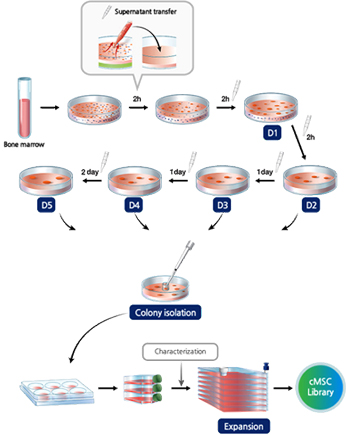
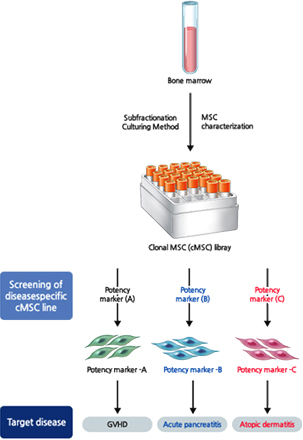
Subfractionation Culturing Method
SCM Lifesciences can isolate and culture high-purity stem cell lines using its original source technology, the Subfractionation Culturing Method.
01
Core Competency
High-purity stem cell line
library construction process
library construction process
Efficacy and disease-specific
stem cell line selection process
stem cell line selection process
-
01Single colony isolationObtained through the Subfractionation Culturing Method single colony isolation process
-
02Single colony-derived cell culture and characterization
- Cell size / density
- Proliferative capacity
- Differentiation ability
- Marker expression
- Immune regulation ability
-
03High-potency cell line selection processEfficacy indicator expression confirmation
-
04Disease-specific stem cell line selection processPerform disease-specific by pre-clinical efficacy evaluation using both animal and cell models
02
Competitive Advantage
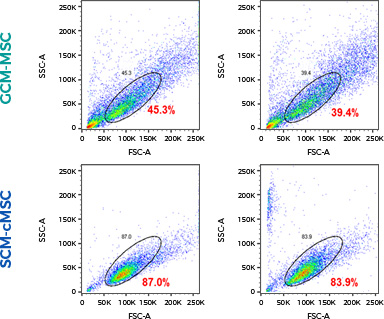
Compared to conventional gradient centrifugation-derived
stem cells (GCM-MSC) Stem cells derived from
the Subfractionation Culturing Method (SCM-cMSC)
High cell density dispersion
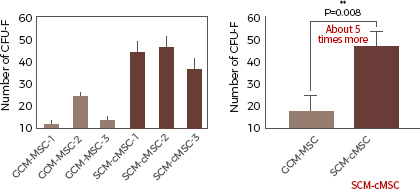
separated by conventional gradient centrifugation
(GCM-MSC) Separated from adult stem cells
by a Subfractionation Culturing Method
The ability to form a cell group of adult stem
cells is more than 5 times higher
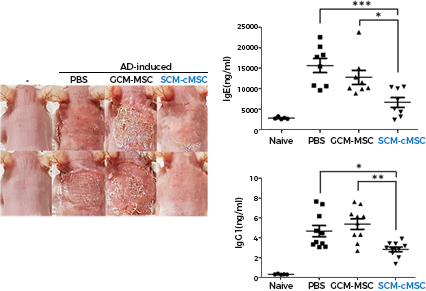
Compared to conventional gradient centrifugation-derived
stem cells (GCM-MSC)
Subfractionation Culturing Method-derived stem cells (SCM-cMSC)
Excellent disease treatment efficacy (eg atopic dermatitis)