There's no previous post.
An article on the results of the Phase 1/2 clinical trial of SCM-AGH, a stem cell therapy developed for atopic dermatitis, has been
published in the Journal of Allergy and Clinical Immunology (JACI, IF: 11.3), a leading journal in the field of allergy and clinical immunology.
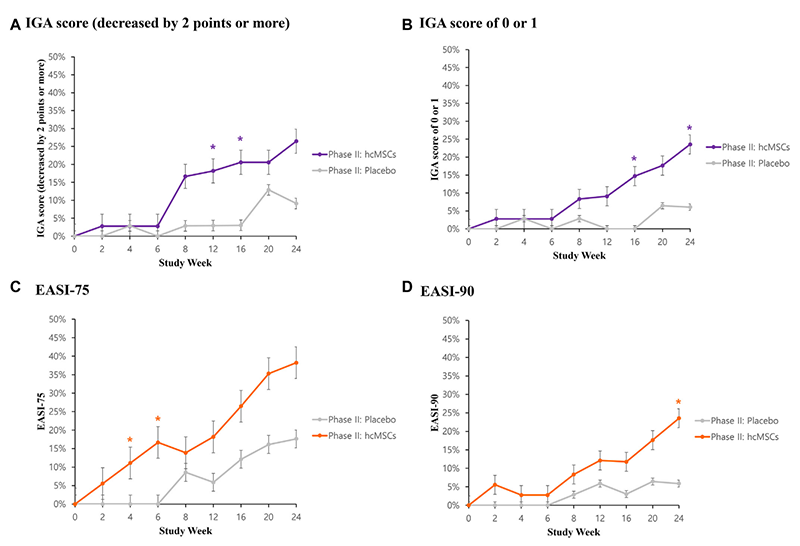
The graph above compares the long-term efficacy of the stem cell therapy for atopic dermatitis between the experimental group and the control group.
The results of the clinical trials for SCM Lifescience’s stem cell therapy for atopic dermatitis have been published in a globally recognized international journal, which is considered the highest authority in the field of immunology. This publication signifies that SCM Lifescience’s research capabilities in stem cell therapy for atopic dermatitis are highly recognized by the international scientific community. Seizing this opportunity, SCM Lifescience plans to actively engage in joint research and technology transfer to expedite the Phase 3 clinical trials of the stem cell therapy for atopic dermatitis, both domestically and internationally.
On the 15th, SCM Lifescience announced that a paper detailing the results of the Phase 1/2 clinical trial of their stem cell therapy for atopic dermatitis, ‘SCM-AGH’, was published in the Journal of Allergy and Clinical Immunology (JACI, IF: 11.3). JACI is a leading journal in the field of allergy and clinical immunology and is recognized internationally as an influential publication among researchers worldwide.
The paper is titled ‘Phase I/II trials of human bone marrow-derived clonal mesenchymal stem cells for treatment of adults with moderate-to-severe atopic dermatitis’.
"Following the Phase 2a study of the acute pancreatitis treatment (SCM-AGH), which is developed with the same substance as the atopic treatment and was well received by the international journal Gastroenterology (IF 33.883) last year, the clinical study of atopic dermatitis has also been published in a top-tier journal, confirming its potential," stated a representative of SCM Lifescience.
Atopic dermatitis is a chronic disease that results in severe itching and skin inflammation, often due to genetic or environmental factors and abnormalities in the immune system. While there are several treatments available for atopic dermatitis, particularly for moderate to severe cases, these treatments often provide short-term relief, have limited effect, or cause side effects. Therefore, there is an urgent need to develop new drugs that are safe and have long-lasting therapeutic effects.
The paper published in JACI by SCM Lifescience analyzed data from patients in clinical trials to evaluate the efficacy of the treatment. This suggests new possibilities for the long-term persistence of therapeutic effects in atopic dermatitis treatment.
According to the paper, the SCM-AGH arm achieved a statistically significant difference in the number of patients achieving a 50% reduction in Eczema Area and Severity Index (EASI) score (EASI-50) at week 12, compared to the placebo arm, meeting the primary efficacy endpoint. Other endpoints, including EASI-75, EASI-90, and Investigator Global Assessment (IGA), showed differences between the treatment and placebo groups up to 24 weeks after SCM-AGH treatment. The treatment also demonstrated safety, with no serious adverse events related to SCM-AGH reported. For more details on the study, please refer to the JACI online publication.
With the publication of the clinical study on atopic dermatitis stem cell therapy in JACI, proving its global market competitiveness, SCM Lifescience is planning to actively conduct a large-scale global Phase 3 clinical trial. Specifically, the company will concentrate on aggressively marketing SCM-AGH to global pharmaceutical companies and establishing partnerships. Handok, our domestic partner, is anticipated to announce whether it will proceed with the Phase 3 clinical trial of SCM-AGH by the end of this month.
Separately, the development of SCM-AGH's acute pancreatitis treatment has shifted to advanced regenerative medicine clinical research, and we have already secured a number of medical institutions to conduct research on SCM-AGH for acute pancreatitis patients. The company plans to initiate research on actual patients after receiving approval from the Ministry of Health and Welfare's Advanced Regenerative Medicine and Advanced Biologics Review Committee as well as the Ministry of Food and Drug Safety (MFDS).
Hyung Nam Oh, Senior Vice President and Acting CEO of SCM Lifescience, stated, “The results of the clinical trial and the publication of the paper represent a significant advancement in the development of a treatment for atopic dermatitis.”
“In the global pharmaceutical and biotechnology market, there is a heightened interest in the development of therapeutics for inflammatory and autoimmune diseases. The technology transfer of actual inflammation modulators and immune system modulators is actively taking place among Big Pharma,” said a representative. “SCM Lifescience’s new drug pipeline meets these two conditions, demonstrating therapeutic efficacy in clinical studies and being published in top global journals, thereby increasing the potential for technology transfer.”
“The recent issue regarding the largest shareholder’s sale of management rights will naturally be resolved when the clinical research results of stem cell therapeutics, isolated by the layer separation culture method - an original stem cell isolation technology developed by our founder, Sun U. Song - are realized, and the Phase 2 clinical results of graft-versus-host disease are successfully announced in the second half of this year.” “In order to increase shareholder value, the management will actively expand the global market by demonstrating R&D achievements on the excellence of stem cells isolated by our proprietary technology.”